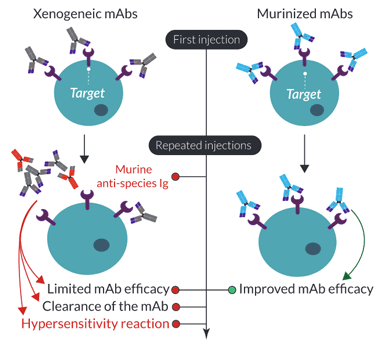
Importance of mAb murinization for in vivo mouse models
Cytokines
• Anti-mIL-1α
• Anti-mIL-1β
• Anti-mIL-2
• Anti-mIL-6
• Anti-mIL-13
• Anti-mIL-28B
Immune checkpoints
• Anti-mCTLA-4
• Anti-mGITR
• Anti-mPD-1
• Anti-mPD-L1
• Anti-mTIGIT
Lymphocyte markers
• Anti-mCD3
• Anti-mCD4
• Anti-mCD8
• Anti-mCD20
• Anti-mCD25
Tumor-associated antigens
• Anti-mgp75
Murine isotype controls
• Mouse IgG1 Control
• Mouse IgG1e3 Control
• Mouse IgG2a Control
In 1985, the FDA approved the first monoclonal antibody (mAb) for clinical use. This mAb was an anti-CD3 antibody for the treatment of organ allograft rejection1. Being of mouse origin, all patients administered with this mAb developed a human anti‑mouse immunoglobulin (Ig) antibody (HAMA) response, significantly limiting its use2. This response leads to the inactivation and clearance of the circulating mAb, causing a loss of treatment efficacy, and in some cases, severe hypersensitivity reactions3. To overcome this limitation, the CD3 mAb was humanized by engineering the complementarity-determining regions (CDRs) into a human backbone4. Currently, all clinically approved mAbs are either humanized or ‘fully’ human through the use of various technologies (e.g. humanized mice)1.
A similar unwanted ‘anti-antibody’ response has been reported in mice upon repeated administration of non-murine, or xenogeneic (e.g. rat/hamster) mAbs. This mouse immune response not only clears the administered mAb but can also cause a lethal hypersensitivity reaction5-7. The use of xenogeneic mAbs for in vivo preclinical research can have significant effects on the assessment of optimal dosing, timing, and repeated injections for the initial in-human trials. Therefore, similar technology used to optimize human mAbs has been applied to mAbs for in vivo mouse research. Firstly, mAbs can be ‘murinized’ through the complete replacement of the non-murine constant regions with mouse Ig sequences, resulting in chimeric mAbs. Importantly, this type of engineering has been shown to not only prolong the circulation of the administrated mAb but also significantly decrease the hypersensitivity reaction upon repeated injections7. Further murinization can be achieved through the murine grafting of the variable CDRs. However, similar to the process for humanization, it is quite complex, difficult to achieve, and can generate a mAb with lower affinity. Ideally, fully murine mAbs can be generated by targeting a gene of interest in knockout (KO) mice. However, this is difficult if the target knockout is embryonically lethal.
Through the use of recombinant technology, mAbs for in vivo research can be significantly improved through the murinization of the backbone and/or the replacement of the isotype for optimal effector function.
Mouse anti-mouse immune checkpoint mAbs
InvivoGen is developing a growing collection of recombinant mouse anti-mouse monoclonal antibodies (mAbs) for in vivo use, some of which are targeting the immune checkpoints (ICs) PD-1, PD-L1, and CTLA-4. These mAbs have been specifically engineered to limit their immunogenicity to maintain constant efficacy upon repeated injections in mice.
Key Features of InvivoGen's InvivoFit™ IC mAbs
- mAb sequence is 85% to 100% murine
- Filter-sterilized (0.2 μm), with an endotoxin level <1 EU/mg
- Suitable for parenteral delivery in mice (azide-free)
- Low aggregation < 5%
- Produced in animal-free facilities with serum-free media
- Functionally validated in mouse tumor models in vivo
- Isotype controls available (Anti-β-Gal mAbs)
Why recombinant?
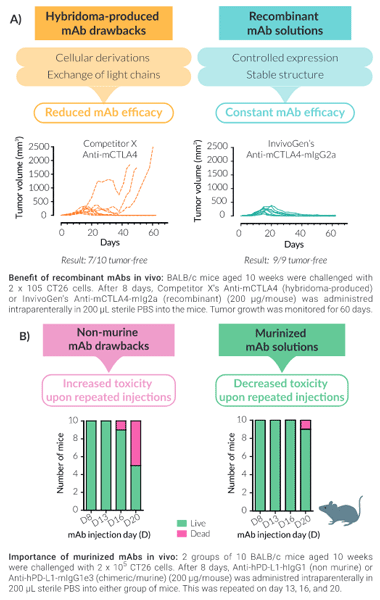
Unlike most IC mAbs on the market that are produced in hybridomas, InvivoGen's recombinant mouse anti-mouse IC mAbs are expressed and produced in CHO cells (virus-free status confirmed), ensuring structural stability. Production of mAbs recombinantly guarantees the highest purity and batch-to-batch consistency (Fig A). They are provided in an InvivoFit™ grade, a high-quality standard specifically adapted for in vivo studies.
Why use murinized mAbs in vivo?
Two major pitfalls encountered in murine in vivo studies upon repeated mAb injections are:
1) decreased antibody performance and,
2) anti‑species antibody generation (if not of mouse origin).
InvivoGen has limited these pitfalls for you by murinizing the non-murine constant regions of anti-human/rat IC mAbs with mouse IgG sequences for reduced toxicity in vivo (Fig B). Additionally, the isotype has been engineered for optimal IC function, for example, InvivoGen's mIgG1e3 isotype contains a D265A mutation, resulting in the complete loss of cytolytic effector function.
InvivoGen's collection includes mAbs targeting:
- Cytokines: mIL-1α, mIL-2, mIL-1β, mIL-6, mIL-13, and mIL-28B
- Immune checkpoints: mCTLA-4, mGITR, mPD-1, mPD-L1, and mTIGIT
- Lymphocyte markers: mCD20, mCD25, mCD3, mCD4, and mCD8
- Tumor-associated antigens: mgp75
- E. coli β-galactosidase: Isotype controls
References :
1. Almagro, J.C. et al. 2017. Progress and Challenges in the Design and Clinical Development of Antibodies for Cancer Therapy. Front Immunol 8, 1751.
2. Kimball, J.A. et al. 1993. OKT3 antibody response study (OARS): a multicenter comparative study. Transplant Proc 25, 558-560.
3. Shawler, D.L. et al. 1985. Human immune response to multiple injections of murine monoclonal IgG. J Immunol 135, 1530-1535.
4. Adair, J.R. et al. 1994. Humanization of the murine anti-human CD3 monoclonal antibody OKT3. Hum Antibodies Hybridomas 5(1-2):41-7.
5. Mall, C. et al. 2016. Repeated PD-1/PD-L1 monoclonal antibody administration induces fatal xenogeneic hypersensitivity reactions in a murine model of breast cancer. Oncoimmunology 5, e1075114.
6. Murphy, J.T. et al. 2014. Anaphylaxis caused by repetitive doses of a GITR agonist monoclonal antibody in mice. Blood 123, 2172-2180.
7. Belmar, N.A. et al. 2017. Murinization and H Chain Isotype Matching of the Anti-GITR Antibody DTA-1 Reduces Immunogenicity-Mediated Anaphylaxis in C57BL/6 Mice. J Immunol 198, 4502-4512.