c-di-GMP VacciGrade™
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
c-di-GMP VacciGrade™ Cyclic diguanylate monophosphate - STING agonist |
Show product |
1 mg |
vac-nacdg
|
|
Cyclic diguanylate monophosphate - STING agonist
Cyclic diguanylate monophosphate (c-di-GMP) is a cyclic dinucleotide (CDN) produced by bacteria in which it functions as an essential second messenger. It controls motility, biofilm formation, and bacterial pathogenicity.
CDNs have been shown to increase vaccine potency [1]. They activate innate immunity by directly binding the endoplasmic reticulum-resident receptor STING (stimulator of interferon genes), activating a signaling pathway that induces the expression of interferon-β (IFN-β) and also nuclear factor-κB (NF-κB) dependent inflammatory cytokines [2, 3].
c-di-GMP exerts strong adjuvant activities when delivered by the mucosal route [4, 5]. It elicits a balanced Th1/Th2 and Th17 response, which is crucial against intracellular pathogens [6].
c-di-GMP VacciGrade™ is a high-quality pre-clinical grade.
c-di-GMP VacciGrade™ is for research use only, and not for human or veterinary use.
![]() Read our review on STING: Deciphering the STING Paradox
Read our review on STING: Deciphering the STING Paradox
References:
1. Dubensky TW. et al., 2013. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Therapeutic Advances in Vaccines 1(4): 131.
2. Jin L. et al., 2011. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J Immunol. 187(5):2595.
3. Burdette DL. et al., 2011. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 478(7370):515.
4. Neuhaus V. et al., 2014. A new adjuvanted nanoparticle-based H1N1 influenza vaccine induced antigen-specific local mucosal and systemic immune responses after administration into the lung. Vaccine. 32(26):3216.
5. Blaauboer SM. et al., 2014. MPYS/STING-mediated TNF-α, not type I IFN, is essential for the mucosal adjuvant activity of (3'-5')-cyclic-di-guanosine-monophosphate in vivo. J Immunol. 192(1):492.
6. Madhun AS. et al., 2011. Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine.29(31):4973.
Specifications
Description: STING agonist VacciGrade™
Synonym: Cyclic di-guanylate monophosphate, c-di-GMP sodium salt
CAS number: 2222132-40-1 / 61093-23-0 (free acid)
Formula: C20H22N10O14P2•2Na
Molecular weight: 734.38 g/mol
Purity: ≥ 95% by LC/MS
Solubility: 50 mg/ml in water
Quality control:
- Sterility guaranteed
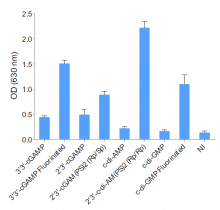
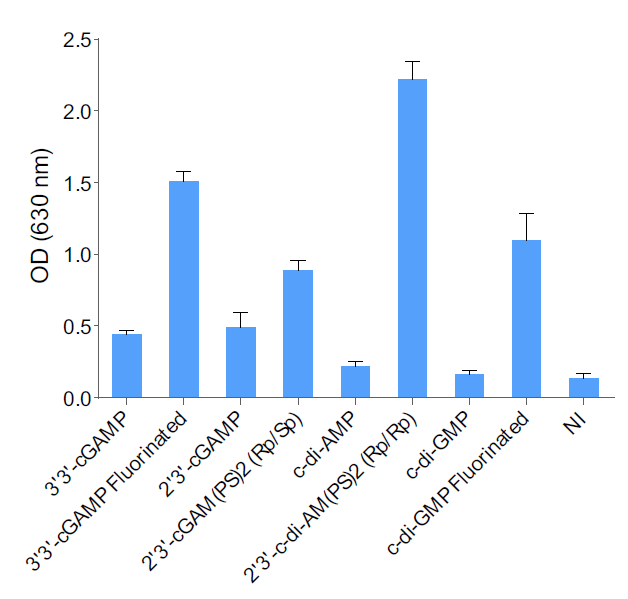
- The absence of bacterial contamination (lipoproteins & endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cells
- Endotoxin level < 5 EU/mg (determined using the HEK-Blue™ LPS Detection Kit 2)
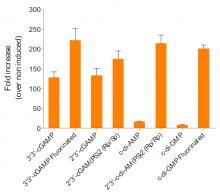
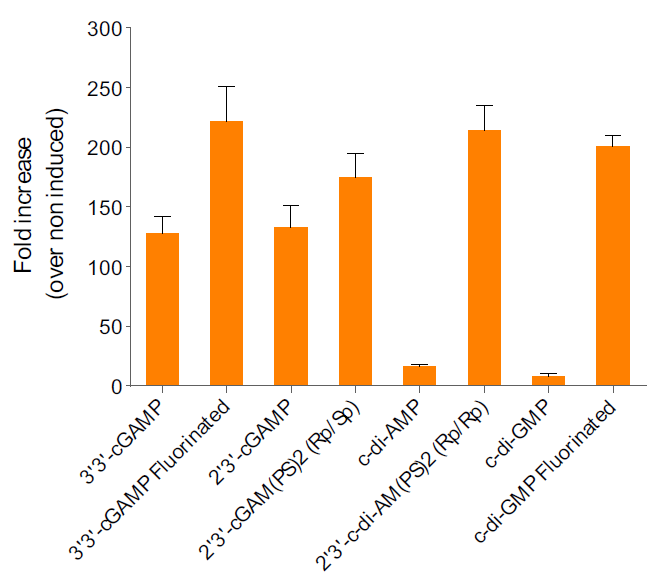
- The ability of c-di-GMP VacciGrade™ to induce type I interferon (IFN) has been confirmed in cellular assays.
Back to the top
Contents
- 1 mg of lyophilized c-di-GMP VacciGrade™
- 10 ml sterile endotoxin-free physiological water (NaCl 0.9%)
![]() c-di-GMPVacciGrade™ is shipped at room temperature
c-di-GMPVacciGrade™ is shipped at room temperature
![]() Stored at -20°C.
Stored at -20°C.
![]() Lyophilized product is stable for 1 year when properly stored.
Lyophilized product is stable for 1 year when properly stored.
VacciGrade™
VacciGrade™ is a high-quality pre-clinical grade. VacciGrade™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room*. The absence of bacterial contamination is assessed by a sterility test using a pharmacopeia-derived assay. The level of bacterial contaminants (endotoxins and lipoproteins) in each lot is verified using a LAL assay and/or a TLR2 and TLR4 reporter assay.
*Except for LPS VacciGrade™, which is prepared in a laminar flow hood dedicated to LPS.