R848 VacciGrade™
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
R848 VacciGrade™ Imidazoquinoline compound -TLR7/8 agonist |
Show product |
5 mg |
vac-r848
|
|
TLR7/TLR8 Agonist - Imidazoquinoline compound | Th1 response

TLR7/TLR8 activation with R848
 InvivoGen also offers:
InvivoGen also offers:
• TLR reporter cells: HEK293, RAW, THP-1 cells
• TLR research tools: Antibodies, Inhibitors, etc.
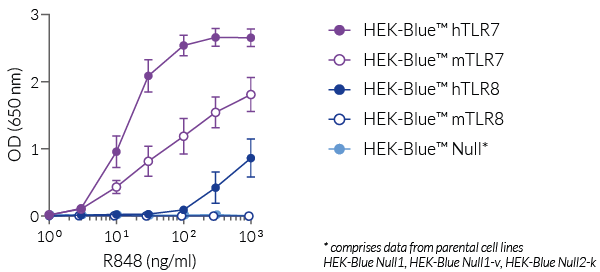
R848 (Resiquimod), an imidazoquinoline, is a dual TLR7 and TLR8 guanosine derivative with potent antiviral activity [1-3]. It induces differential TLR7 and/or TLR8 responses in human and murine immune cells. TLR7 and TLR8 are endosomal pattern recognition receptors that play an important role in the antiviral immune response.
Mode of action
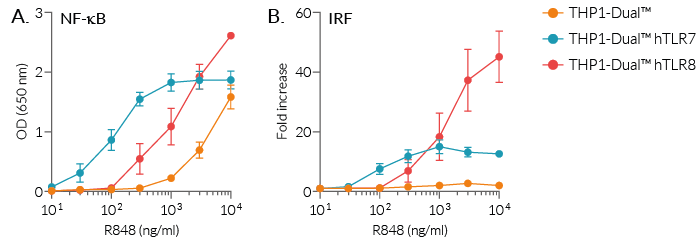
R848 (Resiquimod), a low molecular weight synthetic molecule, acts as a selective activating ligand for both TLR7 and TLR8 in humans but only TLR7 in mice. It activates immune cells via the TLR7/TLR8 MyD88-dependent signaling pathway with the subsequent activation of the transcription factors NF-κB and interferon regulatory factor (IRF) [2, 3]. This ultimately leads to the production of pro-inflammatory cytokines and type I interferons.
Unlike other commercially available R848 preparations, InvivoGen's R848 VacciGrade™ is water-soluble, controlled for TLR7/8 potency and TLR4/TLR2 contamination, and available in a high-quality, pre-clinical grade.
In addition, InvivoGen offers R848 (Resiquimod) in a standard grade for in vitro experiments.
R848 VacciGrade™ is a high-quality pre-clinical grade.
Imiquimod VacciGrade™ is for research use only, and not for human or veterinary use.
Key features of R848
- Agonist of hTLR7 and hTLR8
- Agonist of mTLR7
- Each lot is highly pure (≥95%) and functionally tested
- Standard R848 is also available for in vitro assays
![]() Read our review about TLR7 and TLR8.
Read our review about TLR7 and TLR8.
References:
1. Vanwalscappel B. et al., 2018. Toll-like receptor agonist R848 blocks Zika virus replication by inducing the antiviral protein viperin. Virology 522:199-208.
2. Hemmi H. et al., 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 3(2):196-200.
3. Jurk M. et al. 2002. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R848. Nat Immunol. 3(6):499.
Specifications
Description: TLR7/8 agonist VacciGrade™
Polarization of adaptive immune response: Th1 response
CAS number: 144875-48-9 (free base)
Working concentration: 10 - 100 μg/mouse
Solubility: 1 mg/ml in physiological water
Quality control:
- Sterility guaranteed
- The absence of bacterial contamination (lipoproteins & endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cells
- Endotoxin level < 1 EU/mg (measurement by kinetic chromogenic LAL assay)
Contents
R848 VacciGrade™ is provided lyophilized:
- 5 mg of sterile lyophilized R848 VacciGrade™.
- 10 ml sterile endotoxin-free physiological water (NaCl 0.9%).
![]() R848 VacciGrade™ is shipped at room temperature
R848 VacciGrade™ is shipped at room temperature
![]() R848 VacciGrade™ should be stored at 4°C or -20°C.
R848 VacciGrade™ should be stored at 4°C or -20°C.
![]() Lyophilized product is stable 1 year when properly stored.
Lyophilized product is stable 1 year when properly stored.
Upon resuspension, prepare aliquots of R848 VacciGrade™ and store at -20°C for long term storage.
Resuspended product is stable 6 months when properly stored.
Avoid repeated freeze-thaw cycles.
Back to the topVacciGrade™
VacciGrade™ is a high-quality pre-clinical grade. VacciGrade™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room*. The absence of bacterial contamination is assessed by a sterility test using a pharmacopeia-derived assay. The level of bacterial contaminants (endotoxins and lipoproteins) in each lot is verified using a LAL assay and/or a TLR2 and TLR4 reporter assay.
*Except for LPS VacciGrade™, which is prepared in a laminar flow hood dedicated to LPS.
Details
R848 (resiquimod), a small molecular weight imidazoquinoline compound, is an immune response modifier with potent antiviral and antitumor activities [1].
R848 is being evaluated as an adjuvant in FDA-approved clinical vaccine trials. R848 immune properties result from its ability to induce the production of pro-inflammatory cytokines through the activation of Toll-like receptor (TLR)-7 and TLR8 [2].
In vitro and in vivo studies have shown that R848 promotes the secretion of Th1 cytokines, including IFN-γ, IFN-α, IL-12 and TNF-α [3-7].
R848 is capable of skewing antibody responses toward a Th1 IgG2a response and away from a Th2 IgE response, a feature mediated in part by IFN-α and IL-12.
Unlike most adjuvants, R848 can be administered by a different route than the antigen, suggesting that it does not produce a depot effect.
Preclinical studies in mice have shown that R848 is able to promote adaptive immune responses to codelivered antigens and provide protection against live infection challenges [4, 6, 8, 9].
1. Stanley MA., 2002. Imiquimod and the imidazoquinolines: mechanism of action and therapeutic potential. Clin Dermatol 27:571–7.
2. Hemmi H. et al., 2002. Small anti-viralcompounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200.
3. Wagner tL. et al., 1999. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell. Immunol. 191, 10, 1999.
4. Vasilakos Jp. et al., 2000. Adjuvant activities of immune response modifier R-848: comparison with CpG ODN. Cell. Immunol. 204:64-74.
5.Thomsen L. et al., 2004. Imiquimod and resiquimod in a mouse model: adjuvants for DNA vaccination by particle-mediated immunotherapeutic delivery. Vaccine 22:1799-1809.
6. Baldwin sL. et al., 2009. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine 27:3063-3071.
7. Ma Y. et al., 2010. Assessing the immunopotency of Toll-like receptor agonists in an in vitro tissue engineered immunological model. Immunology 130:374-387.
8. tomai MA. et al., 2000. The immune response modifiers imiquimod and R-848 are potent activators of B lymphocytes. Cell. Immunol. 203:55-65.
9. Zhang WW. & G. Matlashewski. 2008.Immunization with a Toll-like receptor 7 and/or 8 agonist vaccine adjuvant increases protective immunity against Leishmania major in BALB/c mice. Infect. Immun. 76:3777-3783.