Imiquimod VacciGrade™ (R837)
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Imiquimod VacciGrade™ Imidazoquinoline compound -TLR7 agonist |
Show product |
5 mg |
vac-imq
|
|
TLR7 Agonist - Imidazoquinoline compound

Activation of TLR7 by Imiquimod
 InvivoGen also offers:
InvivoGen also offers:
• TLR reporter cells: HEK293, RAW, THP-1 cells
• TLR research tools: Antibodies, Inhibitors, etc.
Imiquimod (R837), an imidazoquinoline amine analog to guanosine, is an immune response modifier with potent indirect antiviral activity. It is an FDA-approved treatment for external genital warts, basal cell carcinoma, and bladder cancer. Imiquimod is a specific agonist for human and mouse Toll-like receptor 7 (TLR7). TLR7 and TLR8 are endosomal pattern recognition receptors that play an important role in the antiviral immune response [1].
Mode of action
The antiviral activity of imiquimod was first shown in guinea pigs infected with herpes simplex virus [2]. Imiquimod is now an FDA-approved treatment for some cancers as well as external genital warts caused by human papillomavirus infection. This low molecular synthetic molecule induces the production of cytokines such as IFN-α. Unlike R848, Imiquimod activates only TLR7 but not TLR8 [3]. This activation is MyD88-dependent and leads to the induction of the transcription factor NF-κB [4].
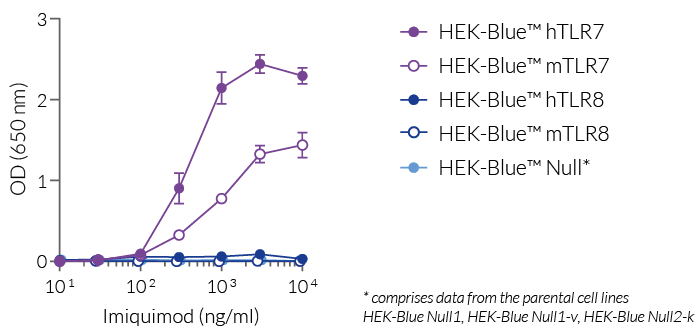
Imiquimod is a human and mouse TLR7-specific agonist, as verified by using our HEK-Blue™ reporter cell lines expressing human or mouse TLR7 or TLR8 (see figure). It is less potent than Gardiquimod™, another TLR7-specific ligand that does not activate TLR8. Even at high concentrations (>10 µg/ml), imiquimod does not activate human or mouse TLR8.
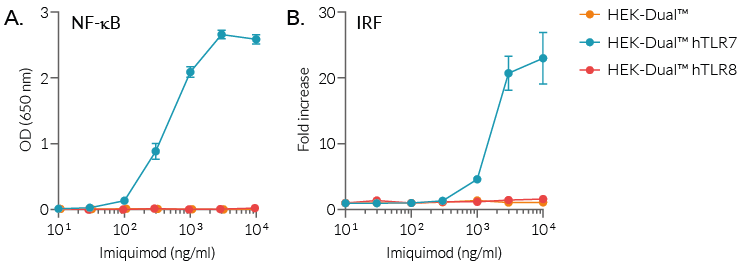
Moreover, imiquimod is able to activate hTLR7-dependent NF-κB and IRF pathways, as assessed using our HEK-Dual™ reporter cell lines expressing two reporter genes, for the NF-κB-inducible SEAP and IRF-inducible Lucia luciferase, as well as hTLR7 or hTLR8 (see figure).
Unlike other commercially available imiquimod products, InvivoGen's Imiquimod (R837) is validated for TLR7/TLR8 potency, tested to ensure the absence of TLR2 or TLR4 contamination, and available in a high-quality, pre-clinical grade. InvivoGen also offers imiquimod (R837) in a standard grade for in vitro experiments.
Imiquimod VacciGrade™ is for research use only, and not for human or veterinary use.
Key features of Imiquimod
- Specific activator of human and mouse TLR7
- Does not activate TLR8
- Each lot of imiquimod is highly pure (≥95%) and functionally tested
- Standard imiquimod is also available for in vitro assays
References:
1. Georg P. & Sander L.E., 2019. Innate sensors that regulate vaccine responses. Curr. Op. Immunol. 59:31.
2. Miller RL. et al., 1999. Imiquimod applied topically: a novel immune response modifier and new class of drug. Int J Immunopharmacol. 1999 Jan;21(1):1-14.
3. Lee J. et al., 2003. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: Activation of Toll-like receptor 7. PNAS, 100(11):6646-51.
4. Hemmi H. et al., 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol, 3(2):196-200.
Specifications
Description: TLR7 agonist VacciGrade™
Polarization of adaptive immune response: Th1 response
Formula: C14H16N4•HCl
Molecular weight: 276.8 g/mol
Solubility: 1 mg/ml in physiological water
Working concentration: 10 - 100 μg/mouse
Quality control:
- Sterility guaranteed
- The biological activity of Imiquimod VacciGrade™ has been validated using cellular assays.
- The absence of bacterial contamination (lipoproteins & endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cells
- Endotoxin level < 5 EU/mg (measurement by kinetic chromogenic LAL assay)
Contents
- 5 mg of sterile lyophilized Imiquimod VacciGrade™
- 10 ml sterile endotoxin-free physiological water (NaCl 0.9%)
![]() Imiquimod VacciGrade™ is shipped at room temperature
Imiquimod VacciGrade™ is shipped at room temperature
![]() Stored at -20°C.
Stored at -20°C.
![]() Lyophilized product is stable for 1 year when properly stored.
Lyophilized product is stable for 1 year when properly stored.
Upon resuspension, prepare aliquots of Imiquimod VacciGrade™ and store at -20°C for long term storage.
Resuspended product is stable for 6 months when properly stored.
Avoid repeated freeze-thaw cycles.
Back to the topVacciGrade™
VacciGrade™ is a high-quality pre-clinical grade. VacciGrade™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room*. The absence of bacterial contamination is assessed by a sterility test using a pharmacopeia-derived assay. The level of bacterial contaminants (endotoxins and lipoproteins) in each lot is verified using a LAL assay and/or a TLR2 and TLR4 reporter assay.
*Except for LPS VacciGrade™, which is prepared in a laminar flow hood dedicated to LPS.
Details
Imiquimod
Imiquimod (R837), an imidazoquinoline amine analog to guanosine, is an immune response modifier with potent antiviral and antitumor activities [1].
Imiquimod is approved for the topical treatment of genital warts, basal cell carcinoma, and bladder cancer. Imiquimod exerts its immune-modulating activity by inducing the production of pro-inflammatory cytokines, such as IFN-α and IL-12, leading to the activation of both innate and acquired immunity [2]. This activity of imiquimod is in part due to its capacity to bind to and stimulate Toll-like receptor (TLR) 7, suggesting a potential role of Imiquimod to act as an adjuvant [3, 4]. Preclinical studies in mice have shown the effectiveness of imiquimod in inducing immune responses to immunization using various vaccination strategies [5-7].
Imiquimod has been shown to increase both antibody and cell-mediated immune responsiveness induced by a DNA vaccine. In a genetically engineered mouse model, a DNA vaccine encoding HER2/neu adjuvanted with imiquimod was reported to significantly delay the development of spontaneous mammary tumors [6]. This antitumor effect was accompanied by a significant increase in Ag-specific antibody (Ab) production and cytotoxic T cell activity, as well as a switch from IgG1 to IgG2a Ab isotype, suggesting a Th1 polarization of the immune response.
TLR7 and TLR8
TLR7 and TLR8 are endosomal pattern recognition receptors that share structural homology [8]. Both receptors are activated by single-stranded RNA (ssRNA) molecules, however, they exhibit different ligand-binding specificities and cellular expression patterns suggesting that they have nonredundant specialized roles.
TLR7 is essentially expressed by plasmacytoid dendritic cells (pDCs) but is also found in B cells and other myeloid cells [2] while TLR8 is highly expressed by myeloid cells and is absent from pDCs and B cells [9].
The endosomal distribution of TLR7 and TLR8 allows them to scan for the presence of microbial RNA in the phagocytic cargo. Their activation leads to NF-κB-, AP1-, and interferon regulatory factor (IRF)-mediated production of type I interferons (IFN-α/β) and pro-inflammatory cytokines [9].
Structural analyses have revealed that both TLR7 and TLR8 possess two binding sites (designated as Site 1 and Site 2) which do not share the same specificities.
Site 1 is highly conserved between TLR7 and TLR8 and binds nucleosides (guanosine (G) for TLR7 and uridine (U) for TLR8) or base analogs. The ligand preference for TLR7 and TLR8 is thus explained by the presence of specific residues in Site 1. Site 1 occupancy allows receptor dimerization and signaling.
Site 2 is less conserved and binds ssRNA with U(U) and U(G) motifs, respectively [10, 11]. Of note, ssRNA-binding to Site 2 is not sufficient for the formation of a signaling-competent TLR dimer but it strongly enhances the binding affinity of Site 1 [3, 4]. Thus, TLR7 and TLR8 appear to sense distinct RNA-degradation products rather than full-length ssRNAs [11].
References:
1. Suader DN., 2000. Immunomodulatory and pharmacologic properties of Imiquimod. J Am Acad Dermatol 43: S6−S11.
2. Stanley MA., 2002. Imiquimod and the imidazoquinolines: mechanism of action and therapeutic potential. Clin Dermatol 27:571−577.
3. Hemmi H. et al. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol, 3(2):196-200.
4. Schon MP & Schon M., 2004. Immune modulation and apoptosis induction: two sides of the antitumoral activity of Imiquimod. Apoptosis 9: 291−298.
5. Thomsen LL et al., 2004. Imiquimod and resiquimod in a mouse model: adjuvants for DNA vaccination by particle mediated immunotherapeutic delivery. Vaccine 22: 1799−1809.
6. Smorlesi A. et al.,2005. Imiquimod and S-27609 as adjuvants of DNA vaccination in a transgenic murine model of HER2/neu-positive mammary carcinoma. Gene Ther. 12(17):1324-32.
7. Triozzi PL. et al., 2010. Regulation of the activity of an adeno-associated virus vector cancer vaccine administered with synthetic Toll-like receptor agonists. Vaccine. 28(50):7837-43.
8. Chuang T.H. & Ulevitch R.J., 2000. Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8, and hTLR9. Eur Cytokine Netw, 11:372-8.
9. Georg P. & Sander L.E., 2019. Innate sensors that regulate vaccine responses. Curr. Op. Immunol. 59:31.
10. Zhang Z. et al., 2018. Structural analyses of Toll-like receptor 7 reveal detailed RNA sequence specificity and recognition mechanism of agonistic ligands. Cell Rep. 25:3371.
11. Tanji H. et al., 2015. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat. Struct. Mol. Biol. 22:109.
Chemical structure of imiquimod