LPS-EB VacciGrade™
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
LPS-EB VacciGrade™ Lipopolysaccharide from E. coli 0111:B4 strain - TLR4-based adjuvant |
Show product |
5 x 1e6 EU |
vac-3pelps
|
|
TLR4-based adjuvant - Lipopolysaccharide from Escherichia coli 0111:B4
Lipopolysaccharide (LPS) is a natural adjuvant synthesized by Gram-negative bacteria. It is a potent activator of the immune system through its recognition by Toll-like receptor 4 (TLR4) and the subsequent pro-inflammatory signaling cascade [1]. LPS is composed of three main structures, an O-antigen, core, and lipid A (also known as endotoxin), with each of these playing a different role in the function of LPS [2]. Specifically, lipid A is responsible for the potent adjuvant activity of LPS.
Similar to other TLR-based adjuvants, LPS drives Th1 immunity, however, under certain conditions, low doses of LPS can promote Th2 responses. While LPS is a potent adjuvant, its pyrogenic activity has prevented its clinical use in vaccines [3].
LPS-EB VacciGrade™ is a preclinical grade of a smooth (S)-form lipopolysaccharide (LPS) purified from the Gram-negative bacteria E. coli 0111:B4.
- LPS-EB VacciGrade™ is extracted by successive enzymatic hydrolysis steps and purified by the previously described phenol-TEA-DOC extraction protocol [4]. This process removes contaminating lipoproteins and therefore LPS-EB VacciGrade™ only activates TLR4.
- LPS-EB VacciGrade™ is prepared under strict aseptic conditions.
LPS-EB VacciGrade™ is for research use only, and not for human or veterinary use.
References:
1. Coffman, R.L. et al., 2010. Vaccine adjuvants: putting innate immunity to work. Immunity 33, 492-503.
2. Cochet, F. & Peri, F., 2017. The Role of Carbohydrates in the Lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) Signalling. Int J Mol Sci 18.
3. McAleer, J.P. & Vella, A.T., 2010. Educating CD4 T cells with vaccine adjuvants: lessons from lipopolysaccharide. Trends Immunol 31, 429-435.
4. Hirschfeld M. et al., 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 165(2):618-22.
Specifications
Species: Escherichia coli 0111:B4 strain
Description: TLR4 agonist VacciGrade™
Polarization of adaptive immune response: Th1 response
Solubility: physiological water
Working concentration: 0.1 - 25 µg/mouse
Quality control:
- Sterility guaranteed
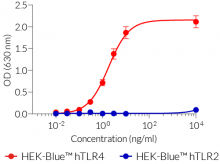
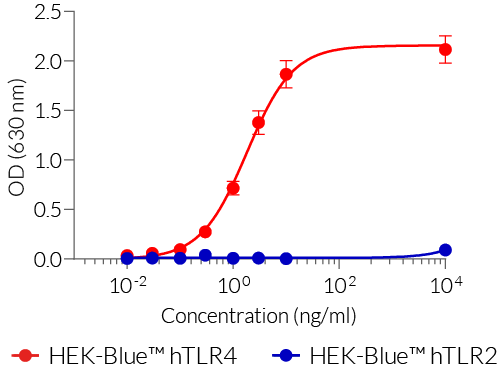
- Presence of other bacterial components is controlled using HEK-Blue™ TLR2 cells
- Endotoxin level is measured using a chromogenic LAL assay and the TLR4 activity is assessed using HEK-Blue™ TLR4 cells.
Contents
LPS-EB VacciGrade™ is provided lyophilized.
-
5 x 106 EU of VacciGrade™ lipopolysaccharide from E. coli 0111:B4 (LPS- EB VacciGrade™)
Note: LPS-EB VacciGrade™ is sterile filtered prior to lyophilization. - 10 ml sterile endotoxin-free physiological water (NaCl 0.9%)
![]() LPS-EB VacciGrade™ is shipped at room temperature.
LPS-EB VacciGrade™ is shipped at room temperature.
![]() Store at -20°C.
Store at -20°C.
![]() Product is stable 1 year when properly stored.
Product is stable 1 year when properly stored.
Upon resuspension, prepare aliquots of LPS-EB VacciGrade™ and store at -20°C.
Resuspended product is stable 6 months when properly stored.
Avoid repeated freeze-thaw cycles
VacciGrade™
VacciGrade™ is a high-quality pre-clinical grade. VacciGrade™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room*. The absence of bacterial contamination is assessed by a sterility test using a pharmacopeia-derived assay. The level of bacterial contaminants (endotoxins and lipoproteins) in each lot is verified using a LAL assay and/or a TLR2 and TLR4 reporter assay.
*Except for LPS VacciGrade™, which is prepared in a laminar flow hood dedicated to LPS.
Details
Lipopolysaccharide (LPS) is a natural adjuvant synthesized by gram-negative bacteria and is a potent activator of the immune system. LPS stimulates the immune response through Toll-like receptor 4 (TLR4) [1].
This recognition involves the binding of LPS with lipopolysaccharide-binding protein (LBP) and subsequently with CD14 which physically associates with a complex including TLR4 and MD2 [2]. Formation of the TLR4-centered LPS receptor complex induces the production of proinflammatory cytokines, such as IL-12 and TNF-α, through the MyD88 pathway.
LPS signaling also involves a MyD88-independent cascade that mediates the expression of IFN-inducible genes.
Similar to other TLR-based adjuvants, LPS drives Th1 immunity [3, 4], although in certain circumstances low-dose inhaled LPS can promote Th2 responses [5]. While LPS is a potent adjuvant, its pyrogenic activity has prevented clinical use of LPS in vaccines. Large quantities of LPS induce the overproduction of cytokines causing septic shock [1].
Intramuscular injection with LPS-EB induced the secretion of the proinflammatory cytokines TNF-α and IL-6 and the Th1-type cytokines, IFN- γ and IL-12 [6]. Cellular assays confirmed that LPS-EB is a potent inducer of dendritic cells activation and maturation via a TLR4 signaling pathway [6].
Most LPS preparations on the market are contaminated by other bacterial components, such as lipoproteins, thus activating TLR2 signaling as well as TLR4 signaling. LPS-EB VacciGrade™ only activates the TLR4 pathway.
1. Poltorak A. et al., 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science, 282(5396): 2085-8.
2. Shimazu R. et al., 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med, 189(11):1777-82.
3. Jamalan M. et al., 2011. Effectiveness of Brucella abortus lipopolysaccharide as an adjuvant for tuberculin PPD. Biologicals: journal of the International Association of Biological Standardization. 39(1); 23-28.
4. Barton G.M. & MedzhitovR., 2002. Control of adaptive immune responses by Toll-like receptors. Curr. Opin. Immunol. 14:380.
5. Iwasaki A. & Medzhitov R., 2004. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 5(10):987-95.
6. Han J. et al., 2014. Characterization of the structure and immunostimulatory activity of a vaccine adjuvant, de-o-acylated lipooligosaccharide. PLoS One. 22;9(1):e85838.